A liver transplant is an operation that replaces a patient’s diseased liver with a whole or partial healthy liver from another person. This article explains the current indications for liver transplantation, types of donor livers, the operation itself, and the immunosuppression that is required after transplantation.
A liver transplant is an operation that replaces a patient’s diseased liver with a whole or partial healthy liver from another person. This article explains the current indications for liver transplantation, types of donor livers, the operation itself, and the immunosuppression that is required after transplantation.
In 2017, UCSF’s liver transplant program earned the highest score for risk adjusted outcomes based on data from the Scientific Registry of Transplant Recipients (SRTR) using SRTR’s new “5-Tier Outcome Assessment” model. Among those institutions receiving the highest ranking nationally, UCSF ranked first in the number of adult liver transplants performed.
Liver Anatomy and Function
The liver is a vital organ, meaning that one cannot live without it. The liver serves many critical functions including metabolism of drugs and toxins, removing degradation products of normal body metabolism (for example clearance of ammonia and bilirubin from the blood), and synthesis of many important proteins and enzymes (such as factors necessary for blood to clot).
Blood enters the liver from two channels, the hepatic artery and the portal vein, bringing nutrients and oxygen to liver cells, also known as hepatocytes, and bile ducts. Blood leaves the liver via the hepatic veins which drain into the inferior vena cava which immediately enters the heart. The liver makes bile, a liquid that helps dissolve fat and eliminate metabolic waste and toxins via the intestine. Each hepatocyte creates bile and excretes it into microscopic channels that join to form bile ducts. Like tributaries joining to form a river, the bile ducts join to form a single “hepatic duct” that brings bile into the intestine.
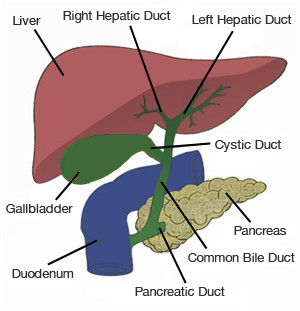 |
The bile ducts join to form a single “hepatic duct” that brings bile into the intestine.
|
Back to top
Who Needs a Liver transplant?
Liver transplantation surgically replaces a failing or diseased liver with one that is normal and healthy. At this time, transplantation is the only cure for liver insufficiency or liver failure because no device or machine reliably performs all of the functions of the liver. People who require liver transplants typically have one of the following conditions.
Acute Liver Failure
Acute liver failure, also known as fulminant hepatic failure, occurs when a previously healthy liver suffers massive injury resulting in clinical signs and symptoms of liver insufficiency. Any number of things can lead to acute liver failure but the most common causes are acetaminophen (Tylenol®) overdose, viral infections (known or yet unknown virus), ingestion of a toxin such as poisonous mushrooms, or an idiosyncratic drug reaction.
The hallmark of this condition is the development of confusion (encephalopathy) within eight weeks after the onset of yellowing of the skin (jaundice). Confusion occurs because toxins typically metabolized by the liver accumulate. Unlike patients with chronic liver disease, who can survive weeks to months to years while awaiting liver transplantation, patients with acute liver failure may die within days if not transplanted. These patients are listed at highest priority (Status I), placing them at the top of local, regional and national waiting lists for a donor liver.
Chronic liver failure
The liver has a remarkable ability to repair itself in response to injury. Nevertheless, repeated injury and repair, typically over many years and even decades, scars the liver permanently. The end stage of scarring is termed cirrhosis and corresponds to the point where the liver can no longer repair itself. Once a person has cirrhosis, he or she may begin to show signs of inadequate liver function. This is termed “decompensated liver disease.” Although medications can decrease the symptoms caused by the liver failure, liver transplantation represents the only permanent cure.
Back to top
Signs and Symptoms of Decompensated Liver Disease
- Gastrointestinal bleeding: As the liver becomes increasingly scarred, the resistance to portal blood flow increases leading to increased pressure in the portal venous system. This portal hypertension necessitates alternative routes for blood to return to the heart. Small veins throughout the abdomen, but outside of the liver, then become enlarged and thin-walled due to the abnormally high amount of blood flowing through them under increased pressure. These fragile veins, called varices, often line portions of the gastrointestinal tract, especially the esophagus and the stomach, and are prone to rupture and bleeding. When bleeding occurs into the intestinal tract, it can be life-threatening.
- Fluid retention: One function of the liver is to synthesize many of the proteins circulating in the bloodstream, including albumin. Albumin and other proteins in the blood stream retain fluid in the vascular space by exerting what is known as an oncotic (or osmotic) pressure. In liver failure, low albumin levels force fluid out of the bloodstream, which cannot be re-absorbed. Fluid therefore accumulates in tissues and body cavities, most commonly, in the abdominal cavity, which is termed “ascites.” Fluid can also accumulate in the legs (peripheral or pedal edema), or in the chest cavity (hydrothorax). Fluid retention is treated first by strict limitation of dietary salt intake, second with medications (diuretics) that force increased salt and water loss through the kidneys and, lastly, by intermittent drainage through insertion of a needle into the abdominal or chest cavity.
- Encephalopathy: Failure of the liver to clear ammonia and other toxins from the blood allows these substances to accumulate. These toxins result in cognitive dysfunction that ranges from disturbed sleep-wake cycle patterns to mild confusion to coma.
- Jaundice: One of the main functions of the liver is to eliminate the degradation products of hemoglobin, the molecule that carries oxygen in our blood. Bilirubin is one of those degradation products processed and excreted by the liver. In liver failure, bilirubin is not cleared from the body and bilirubin levels increase in the blood. The skin and all tissues of the body will then assume a yellow color.
Back to top
Causes of Chronic Liver Injury
Viral Hepatitis
- Hepatitis B: Hepatitis B infection accounts for 5% of all liver transplants performed in the United States but accounts for a larger proportion of liver transplants in other parts of the world, especially Asia and Australia / New Zealand.
- Hepatitis C: This is the most common indication for liver transplantation in the United States, affecting nearly 50% of all liver transplant recipients.
Alcoholic Liver Disease
Liver failure due to alcohol abuse is the second most common indication for liver transplantation in the United States. Most centers require at least a six-month period of abstinence, often within a recognized substance abuse program such as Alcoholics Anonymous, as a condition of listing for transplantation.
Metabolic Liver Disease
Non-alcoholic steatohepatitis (NASH): Deposition of fat within liver cells may result in inflammation that injures and scars the liver. Risk factors for the development of fatty liver and NASH include obesity and metabolic conditions such as diabetes and hyperlipidemia (increased cholesterol). The percentage of patients being transplanted for this condition has increased 35 fold from 2000 to 2005.
Autoimmune Liver Disease
- Autoimmune hepatitis (destruction of the liver by the patient’s own immune system)
- Cholestatic Liver Diseases
- Primary Biliary Cirrhosis (PBC) (destruction of small bile ducts within the liver)
- Primary Sclerosing Cholangitis (PSC) (destruction of bile ducts inside and outside the liver). Seventy percent of patients with PSC also suffer from ulcerative colitis, an autoimmune disorder of the colon.
- Neonatal sclerosing cholangitis (infection and scarring of the bile ducts in the liver of an infant)
- Biliary atresia (absence of bile ducts outside the liver)
- Caroli’s disease (abnormality of the bile ducts within the liver)
- TPN-induced cholestasis. Patients who receive intravenous nutrition, termed total parenteral nutrition (TPN) sometimes develop bile stasis (slowing or stopping of normal bile flow) that can, over time, lead to liver injury and failure.
Genetic Liver Disease
- Hemachromatosis: excess iron deposition in the liver
- Wilson’s disease: abnormal copper metabolism
- Alpha-1 anti-trypsin deficiency: lack of a gene product that limits the activity of trypsin, an enzyme that digests protein. Over time this leads to progressive destruction of the liver and lung.
- Glycogen storage disease (type I, III, IV): an inherited metabolic disorder
- Tyrosinemia: a disorder of tyrosine metabolism
Vascular Liver Disease
Budd-Chiari syndrome is thrombosis (clotting) of the hepatic veins which leads to poor blood flow though the liver.
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is a primary cancer of the liver, meaning that it originates from abnormal liver cells. HCC occurs only rarely in a normal, non-cirrhotic liver. Its incidence is, however, strikingly increased in the background of cirrhosis and, in particular, by certain types of liver disease that lead to cirrhosis (hepatitis B and C, hemachromatosis, and tyrosinemia). Although the cancer first starts within the liver, as it grows it can spread to other organs, a process called metastasis. HCC most frequently spreads to the lungs or to bones. The risk of spread outside of the liver increases with the size of the cancer.
Liver transplantation definitively cures a patient of HCC, provided that the tumor has not spread beyond the liver. Because there are far more people in need of liver transplants than there are available organs, specific guidelines, called the Milan Criteria, have been established to define which patients with HCC are eligible for transplantation. These criteria define limits of tumor number and size that ensure a very low likelihood of cancer spread outside of the liver.
Back to top
Who Are Not Candidates for a Liver Transplant
There are many people with cirrhosis and decompensated liver disease but not all are appropriate candidates for liver transplantation. A patient must be able to survive the operation and the potential post-operative complications, reliably take the medications that prevent rejection and opportunistic infections, comply with frequent clinic visits and laboratory tests, and not engage in activity that would injure the liver, such as drinking alcohol. The conditions listed below are generally considered to be absolute contra-indications to liver transplantation.
- Severe, irreversible medical illness that limits short-term life expectancy
- Severe pulmonary hypertension (mean pulmonary artery pressure greater than 50mmHg)
- Cancer that has spread outside of the liver
- Systemic or uncontrollable infection
- Active substance abuse (drugs and/or alcohol)
- Unacceptable risk for substance abuse (drugs and/or alcohol)
- History of non-compliance, or inability to adhere to a strict medical regimen
- Severe, uncontrolled psychiatric disease
Back to top
Liver Allocation
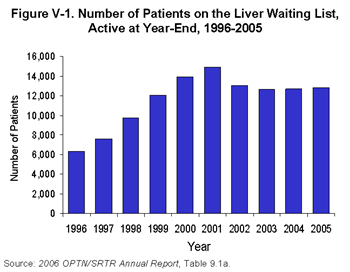
Allocation policy determines how any available organs will be distributed among the many candidates on the waiting list. Over the past five years, the number of patients awaiting a liver transplant has been largely unchanged.
Our current allocation policy is guided by the principles outlined by the Final Rule, issued by the Department of Health and Human Services in March 2000. The Rule stipulates that allocation policy should give primary consideration to the urgency of a recipient’s need for transplantation. This has come to be known as the concept of “sickest first.”
MELD Score
 Much research has gone into trying to understand how to accurately determine how sick a person is from his or her liver disease. A scoring system, called MELD (Model for End-stage Liver Disease), has been identified as highly predictive of the risk of death posed by chronic liver disease.
Much research has gone into trying to understand how to accurately determine how sick a person is from his or her liver disease. A scoring system, called MELD (Model for End-stage Liver Disease), has been identified as highly predictive of the risk of death posed by chronic liver disease.
The MELD score is determined by the results of three objective and readily available laboratory tests:
- Total bilirubin, a measure of jaundice
- Prothrombin time, a measure of clotting ability
- Creatinine, a measure of kidney function. Inputting these three numbers into the following formula yields the actual numerical score.
MELD = 3.8 X log e(total bilirubin [mg/dL]) + 11.2 X log e(INR) + 9.6 X log e(creatinine [mg/dL])
A MELD calculator is used to determine MELD scores.
As a patient’s liver function deteriorates, the laboratory test results increase as will his/her MELD score which will move the patient to a higher position on the waitlist. The patient with the highest MELD score (the sickest patient) is therefore at the top of the list. Lists are, however, organized by blood type. When a donor liver becomes available, the blood type of the donor is determined and the person at the top of the list for that blood group is offered the organ. If that person is too sick or does not accept the liver for whatever reason, the liver is then offered to the next person on the list and so forth until a suitable recipient is found.
Geography and DSAs
Another complicating factor in liver allocation policy is geography. The United States is divided into 11 regions and each region, in turn, is divided into multiple donor service areas (DSAs).
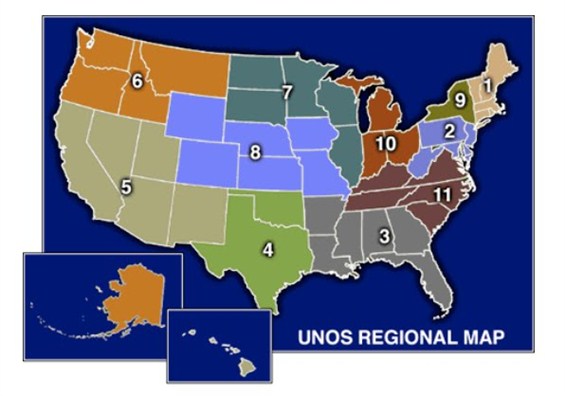 |
| 11 Multiple Donor Service Areas (DSAs) in the U.S. |
The DSAs are the smallest or the “local” unit of organ allocation. Most frequently, organs that are procured from donors in a specific DSA are allocated to candidate recipients within the same DSA. This policy arose as an approach to minimize times for organ transportation and preservation. The Final Rule, however, emphasized the importance of disease severity and discounted the impact of geography in organ allocation policy. Therefore, if there are candidates with the highest acuity and severity of liver disease – those listed as “Status 1” because of acute / fulminant hepatic failure or primary non-function – livers are allocated on a regional or national basis.
Back to top
Types of Organ Donors
Brain dead organ donors
Most livers used for transplantation are obtained from patients that are brain dead. Brain death is usually due to a large stroke or massive trauma to the head from blunt injury (for example, impact to the head from a motor vehicle or a motorcycle accident) or penetrating injury (for example, a gun shot wound). The trauma has stopped all brain function although other organs including the liver may continue to function normally.
There are strict definitions as to what constitutes brain death based on the complete absence of any type of brain function. Because patients that meet criteria for brain death are legally dead, they are appropriate organ and tissue donors. In the United States, the family of someone who is brain dead must provide consent for organ and/or tissue donation. In other countries, such as France, consent for organ donation is presumed and allowed, unless the family objects.
Typically, transplant centers whose patients will be receiving organs from a particular donor will dispatch a team of surgeons to procure the relevant organ. The organ procurement procedure takes place in an operating room in the donor’s hospital. Organs are removed and preserved in a fashion to optimize their condition during the storage and transportation time period. Each procured organ is then transported to the hospitals where the designated recipient awaits.
Cardiac death organ donors
Sometimes a patient suffers a devastating brain injury and carries a dismal neurological prognosis but fails to meet the strict criteria defining brain death in that there is still detectible brain function. In these circumstances, the patient’s family may decide to withdraw life-sustaining medical support with the intention of allowing the patient to die. In this scenario, death is not defined by brain death but rather cardiac death. Organ donation can occur after cardiac death but, again, only if the family gives consent.
Only AFTER the family’s decision to withdraw support may the patient be considered for organ donation after death. Under these circumstances, support is withdrawn, as desired by the family and managed by the patient’s physician, and the patient is allowed to expire. The patient’s physician, someone who is not involved in any aspect of organ transplantation, is present to determine when the heart stops beating and circulation has stopped such that the patient no longer has any signs of life. He or she then declares the patient’s death.
An urgent operation is then performed to preserve and remove organs for transplantation. This mode of cardiac death, in contrast to brain death, results in increased injury to the organs during two time periods. The first period is that between withdrawal of life support and death. As the donor’s breathing and circulation deteriorates, the organs may no longer be receiving sufficient oxygen. The second time period constitutes the minutes immediately after death and until the organs are flushed with preservation solution and cooled. As a result, livers procured from cardiac death donors are associated with an increased risk of primary non function or poor early organ function, hepatic artery thrombosis, and biliary complications (see Complications section).
Living Donors
Although each person has only one liver and would die without it, it is possible to donate a portion of the liver for transplantation into another individual. The segmental anatomy (see figure below) allows surgeons to create grafts of varying size, depending upon the recipient’s requirement for liver tissue. The partial livers in both the donor and the recipient will grow to provide normal liver function for both individuals.
Historically, this procedure was developed to enable transplantation of children as it was difficult to find suitable livers from deceased donors for this group. To transplant a child, typically a graft comprised of segments 2 and 3, together known as the left lateral segment representing 20-25% of the whole liver volume, is used.
Transplantation of an older child or perhaps a petite adult, however, may require segments 2, 3, and 4, together known as the left lobe and representing approximately 40% of the whole liver volume. During the past decade, however, this technique has been further extended to enable transplantation of adults using the right lobe, segments 5, 6, 7, and 8, which account for approximately 60% of the total liver volume. An adult-to-adult living donor liver transplant is highly complex and technically challenging procedure that carries a significant risk for both the donor and the recipient.
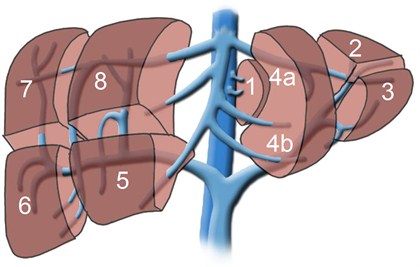 |
| The liver is divided into eight (8) segments reflecting the eight (8) major divisions of the portal vein and the bile duct. Image provided courtesty of Royal College of Surgeons of Ireland (RCSI) under Creative Common License.http://www.healcentral.org/ |
Back to top
The Liver Transplant Operation
A liver transplant involves the removal of and preparation of the donor liver, removal of the diseased liver, and implantation of the new organ. The liver has several key connections that must be re-established for the new organ to receive blood flow and to drain bile from the liver. The structures that must be reconnected are the inferior vena cava, the portal vein, the hepatic artery, and the bile duct. The exact method of connecting these structures varies depending on specific donor and anatomy or recipient anatomic issues and, in some cases, the recipient disease.
For someone undergoing liver transplantation, the sequence of events in the operating room is as follows:
- Incision
- Evaluation of the abdomen for abnormalities that would preclude liver transplantation (for example: undiagnosed infection or malignancy)
- Mobilization of the native liver (dissection of the liver attachments to the abdominal cavity)
- Isolation of important structures (the inferior vena cava above, behind, and below the liver; the portal vein; the common bile duct; the hepatic artery)
- Transection of the above mentioned structures and removal of the native, diseased liver. (Figure 7)
- Sewing in the new liver: First, venous blood flow is re-established by connecting the donor’s and the recipient’s inferior vena cava and portal veins. Next, arterial flow is re-established by sewing the donor’s and recipient’s hepatic arteries. Finally, biliary drainage is achieved by sewing the donor’s and recipient’s common bile ducts.
- Ensuring adequate control of bleeding
- Closure of the incision
Surgical Complications
As with any surgical procedure, complications related to the operation may occur, in addition to the many possible complications that may happen to any patient who is hospitalized. Some of the problems specific to liver transplantation that may be encountered include:
Primary non-function or poor function of the newly transplanted liver occurs in approximately 1-5% of new transplants. If the function of the liver does not improve sufficiently or quickly enough, the patient may urgently require a second transplant to survive.
- Hepatic artery thrombosis, or clotting of the hepatic artery (the blood vessel that brings oxygenated blood from the heart to the liver) occurs in 2-5% of all deceased donor transplants. The risk is doubled in patients who receive a living donor transplant. The liver cells themselves typically do not suffer from losing blood flow from the hepatic artery because they are primarily nourished by blood by the portal blood flow. In contrast, the bile ducts depend strongly on the hepatic artery for nutrition and loss of that blood flow may lead to bile duct scarring and infection. If this occurs, then another transplant may be necessary.
- Portal vein thrombosis or clotting of the large vein that brings blood from the abdominal organs (the intestines, the pancreas, and the spleen – the organs that belong to the portal circulation) to the liver occurs infrequently. This complication may or may not require a second liver transplant.
- Biliary complications: In general, there are two types of biliary problems: leak or stricture. Biliary complications affect approximately 15% of all deceased donor transplants and up to 40% of all living donor transplants.
- Biliary leak means that bile is leaking out of the bile duct and into the abdominal cavity. Most frequently, this occurs where the donor and recipient bile ducts were sewn together. This is often treated by placing a stent, or plastic tube, across the connection through the stomach and small intestine and then allowing the connection to heal. In the case of living donor or split liver transplants, bile can also leak from the cut edge of the liver. Typically, a drain is placed and left during the transplant operation along the cut edge to remove any bile that may leak. As long as the bile does not collect in the abdomen, the patient does not become ill. Leaks will often heal with time, but may require additional treatment procedures.
- Biliary stricture means narrowing of the bile duct, resulting in relative or complete blockage of the bile flow and possible infection. Most frequently, the narrowing occurs at a single site, again where the donor and recipient ducts are sewn together. This narrowing can often be treated by dilating the narrowed area with a balloon and/or inserting a stent across the stricture. If these methods are unsuccessful, surgery is often done to create a new connection between the liver’s bile duct and a segment of intestine. Rarely, biliary strictures occur at multiple or innumerable sites throughout the biliary tree. This occurs most frequently because the biliary tree was poorly preserved during the period when the liver was not in either the donor or recipient circulation. Livers procured from cardiac death donors are at higher risk than those from brain dead donors. Alternatively, diffuse biliary strictures may occur if the biliary tree has inadequate blood supply because of an abnormality with the hepatic artery.
- Bleeding is a risk of any surgical procedure but a particular risk after liver transplantation because of the extensive nature of the surgery and because clotting requires factors made by the liver. Most transplant patients bleed a minor amount and may get additional transfusions after the operation. If bleeding is substantial or brisk, return to the operating room for control of bleeding is often necessary. In general, approximately 10% of transplant recipients will require a second operation for bleeding.
- Infection – Infections can occur during the healing of the wound created by any operation. Liver transplant recipients are also at risk for infections deep within the abdomen, particularly if there is a collection of blood or bile (from a bile leak). The immunosuppressive medications along with the history of liver failure increase the liver transplant recipient’s risk for developing an infection after transplantation.
Immunosuppression
The human body has developed a very sophisticated series of defenses against bacteria, viruses, and tumors. The machinery of the immune system has evolved over millions of years to identify and attack anything that is foreign or not “self.” Unfortunately, transplanted organs fall into the category of foreign, not self. A number of drugs are given to transplant recipients to dampen the responses of their immune system in an attempt to keep the organ safe and free of immunologic attack. If the immune system is not sufficiently weakened, then rejection – the process by which the immune system identifies, attacks, and injures the transplanted organ – ensues.
Commonly used drugs to prevent rejection by suppressing the immune system are listed below. They work through different mechanisms to weaken the immune system’s responses to stimuli and are associated with different side effects. As a result, these medications are frequently used in various combinations which increase the overall immunosuppressive effect while minimizing side effects.
- Corticosteroids (methylprednisolone is given intravenously; prednisone is given orally): Corticosteroids are a class of anti-inflammatory agents that inhibit production of cytokines, the signaling molecules produced by cells of the immune system to orchestrate and intensify the immune response. Corticosteroids therefore prevent activation of lymphocytes, the main soldiers of the immune response against transplanted organs. This is thought to prevent T-cell (a subset of lymphocytes) activation in a non-specific manner. Side effects of corticosteroids are broad and include hyperglycemia, hypertension, decreased bone density, and impaired wound healing,
- Calcineurin inhibitors (cyclosporine, tacrolimus): This class of drugs blocks the function of calcineurin, a molecule critical to a very important lymphocyte signaling pathway that triggers the production of multiple cytokines. These drugs, first developed approximately 20 years ago, revolutionized organ transplantation. They substantially reduced the incidence of rejection, improved the longevity of transplanted organs and thereby ushered in the contemporary era of transplantation and immunosuppression. Unfortunately, these drugs come with a significant side effect profile. The most serious toxicity, particularly with long-term use, is kidney injury. Calcineurin inhibitors also raise blood pressure, glucose levels, and cholesterol – and cause tremors and headaches.
- Mycophenolate mofetil (Cellcept®, Myfortic®): This drug is converted in the body to mycophenolic acid, which inhibits the ability of lymphocytes to replicate DNA, the genetic material essential to every cell. If lymphocytes cannot synthesize DNA, then they are unable to divide to generate additional cells. Mycophenolate mofetil, therefore, dampens the immune response by preventing proliferation of lymphocytes. The primary side effects of mycophenolate mofetil affect the intestinal system resulting in stomach upset and/or diarrhea. It can also depress bone marrow function and thereby, reduce blood levels of white cells (infection fighting cells), red cells (oxygen carrying cells), and platelets (clotting agents).
- mTOR inhibitors (sirolimus; everolimus): mTOR stands for mammalian Target Of Rapamycin. mTOR belongs to a family of enzymes known as kinases and is involved in checkpoint regulation of the cell cycle, DNA repair, and cell death. Inhibition of mTOR stops T cells from progressing through the various phases of the cell cycle, leading to cell cycle arrest. Thus, lymphocytes are not able to divide to amplify the immune response. Side effects of mTOR inhibitors include bone marrow depression, poor wound healing, and increased cholesterol levels.
- Antibodies that target the IL-2 receptor, a signaling molecule that amplifies the immune response (basiliximab, daclizumab): T cells, the agents of acute rejection, express increasing amounts of IL2-receptors when they are stimulated. The IL-2 receptor allows ongoing amplification of an immune response. Blockage of this receptor therefore dampens the immune response. These antibodies are most frequently used for a short time period beginning at the time of transplant to provide additional immunosuppression during this period of highest rejection risk. Immediate side effects include fever, rash, cytokine release syndrome, and anaphylaxis. They do appear to increase the risk of infections hen combined with other immunosuppressive medications.
- Antibodies that remove T cells from the circulation (Thymoglobulin®, OKT-3®): These agents are molecules that target different cells of the immune system, bind them, inactivate, and remove them. They can be used at the time of liver transplantation. but more often are used to treat severe rejection or rejection that does not respond to lesser treatment strategies. Immediate side effects of these medications range from fever and rash to cytokine release syndrome resulting in flash pulmonary edema and hypotension. These drugs may also result in increased incidence of PTLD and skin cancers (see below)
- investigational drugs – As our understanding of the immune system improves, researchers have identified new cells, molecules, and pathways that play a role in the body’s response to transplanted organs. Each discovery presents new opportunities in the form of new targets for drug development. Some of these medicines are currently being tested in clinical trials to determine if they are safe and effective for use in transplantation. Future generations of drugs will hopefully be more specific in preventing rejection without interfering significantly with the other functions of the immune system or causing non-immunologic side effects.
Back to top
Rejection
Rejection is a term that is applied to organ dysfunction caused by the recipient’s immune system reaction to the transplanted organ. Injury to the liver is typically mediated by immune cells, T cells or T lymphocytes. Rejection typically causes no symptoms; patients do not feel any differently or notice anything. The first sign is usually abnormally elevated liver laboratory test results. When rejection is suspected, a liver biopsy is performed. Liver biopsies are easily done as a bedside procedure using a special needle that is introduced through the skin. The tissue is then analyzed and inspected under the microscope to determine the pattern of liver injury and also to look for the presence of immune cells.
Acute cellular rejection occurs in 25-50% of all liver transplant recipients within the first year after transplantation with the highest risk period within the first four to six weeks of transplantation. Once the diagnosis is made, treatment is fairly straightforward and generally very effective. The first line of treatment is high dose corticosteroids (see Immunosuppression section). The patient’s maintenance immunosuppression regimen is also escalated to prevent subsequent rejection. A small proportion of acute rejection episodes, approximately 10-20%, does not respond to corticosteroid treatment and are termed “steroid refractory,” requiring additional treatment.
The second line of rejection treatment is strong antibody preparations (see Immunosuppression Section). In liver transplantation, unlike other organs, acute cellular rejection does not generally affect overall chances for graft survival. This is believed to be because the liver has the unique ability to regenerate when injured thereby restoring full liver function.
Chronic rejection occurs in 5% or less of all transplant recipients. The strongest risk factor for the development of chronic rejection is repeated episodes of acute rejection and/or refractory acute rejection. Liver biopsy shows loss of bile ducts and obliteration of small arteries. Chronic rejection, historically, has been difficult to reverse, often necessitating repeat liver transplantation. Today, with our large selection of immunosuppressive drugs, chronic rejection is more often reversible.
Recurrent Disease
Some of the processes that led to the failure of the patient’s own liver can damage the new liver and eventually destroy it. Perhaps the best example is hepatitis B infection. In the early 1990’s, patients who received liver transplants for hepatitis B infection had less than 50% five year survival. The vast majority of these patients suffered from very aggressive reinfection of the new liver by hepatitis B virus. During the 1990’s, however, several drugs and strategies to prevent re-infection and damage of the new liver were developed and instituted widely by transplant centers. These approaches have been highly successful such that recurrent disease is no longer a problem. Hepatitis B, once considered a contra-indication to transplantation, is now associated with excellent outcomes, superior to many of the other indications for liver transplantation.
Currently, our primary problem with recurrent disease is focused on hepatitis C. Any patient that enters transplantation with hepatitis C virus circulating in their blood will have ongoing hepatitis C after transplantation. However, those who have completely cleared their virus and do not have measurable hepatitis C in the blood will not have hepatitis C after transplantation.
Unlike hepatitis B where recurrent disease leading to liver failure occurs very rapidly, recurrent hepatitis C typically causes a more gradual attrition of liver function. Only a small percentage of hepatitis C recipients, approximately 5%, return to cirrhosis and end stage liver disease within two years of transplantation.
Most have more gradually progressive disease such that as many as half will have cirrhosis at approximately 10 years after transplant. Interferon preparations in combination with ribavirin, widely used in pre-transplant hepatitis C patients, can also be prescribed after transplantation. Chances for permanent cure are somewhat lower than treatment before transplantation. Moreover, the treatment is associated with a significant complement of side effects. Recurrent disease is responsible for the fact that hepatitis C liver transplant recipients have worse medium and long-term post-transplant outcomes compared to liver transplant recipients without hepatitis C (Figure 8).
Several other diseases may also recur after transplantation, but typically the disease is mild and only slowly progressive. Primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) both recur approximately 10-20% of the time and, only very rarely, result in recurrent cirrhosis and end stage liver disease. Perhaps the biggest unknown in today’s age is fatty liver disease after transplantation as it is clearly a problem of increasing frequency. Fatty liver disease can occur in those transplanted for NASH but also in patients who were transplanted for other indications and develop risk factors for fatty liver disease. The frequency, trajectory, and prognosis of recurrence of fatty liver disease after transplant and its course are active areas of research.
Opportunistic Infections and Cancer
As previously stated, the immune system’s primary role is to identify and attack anything that is foreign or non-self. The main targets were not intended to be transplanted organs, but rather bacteria, viruses, fungi, and other microorganisms that cause infection. Taking immunosuppression weakens a transplant recipient’s defenses against infection
As a result, transplant recipients are at increased risk to develop not only standard infections that may affect all people but also “opportunistic” infections, infections that only occur in people with compromised immune systems. The changes in the immune system predispose transplant recipients to different infections based on the time relative to their transplant operation.
They can be divided into three periods: month one, months one to six, and beyond six months. During the first month, infections with bacteria and fungi are most common. Viral infections such as cytomegalovirus and other unusual infections such as tuberculosis and pneumocystis carinii are seen within the first six months.
In addition to fighting infection, the immune system also fights cancer. It is believed that a healthy immune system detects and eliminates abnormal, cancerous cells before they multiply and grow into a tumor. It is well-recognized that transplant recipients are at increased risk for developing several specific types of cancers.
Post-Transplant Lymphoprolipherative Disorder (PTLD)
Post-Transplant Lymphoprolipherative Disorder (PTLD) is an unusual type of cancer that arises exclusively in transplant recipients, as suggested by its name. It is almost always associated with Epstein-Barr virus (EBV), the same virus that causes infectious mononucleosis or “the kissing disease.”
The majority of adults have been exposed to EBV, most commonly in their childhood or teenage years. For these patients, EBV-associated PTLD can develop after transplantation because immunosuppression allows the virus to reactivate. In contrast, many children come to liver transplantation without ever having been exposed to EBV. If patients are exposed to EBV after transplantation and therefore under the influence of immunosuppression, they may be unable to control the infection.
PTLD arises in either scenario when EBV-infected B cells (a subset of lymphocytes) grow and divide in an uncontrolled fashion. As it is fundamentally a result of a compromised immune system, the first line of treatment is simply stopping or substantially reducing immunosuppression. While this approach frequently works, it also risks graft rejection which would then necessitate increased immunosuppression. Recently, a drug that specifically eliminates B cells, the cells infected by EBV, has become available.
Today, a common approach is therefore to give this drug, rituximab, in conjunction with less drastic cuts of the immunosuppression drugs. If this approach does not control PTLD, then more conventional chemotherapy drug regimens typically given to treat lymphomas that develop in non-immunosuppressed patients, are used. The majority of PTLD cases can be successfully treated with preservation of the transplanted organ.
Non-Melanoma Skin Cancer (NMSC)
Skin cancers are the most common malignancy in the post-transplant population. The rate of skin cancer in patients who have undergone organ transplantation is 27% at 10 years, reflecting a 25-fold increase in risk relative to the normal population. In light of this substantial risk, it is strongly recommended that all transplant recipients minimize sun exposure.
Moreover, all transplant recipients should be regularly examined to ensure early diagnosis and expeditious treatment of any skin cancer. There is some evidence to suggest that sirolimus, an immunosuppressant in the class of mTOR inhibitors (see Immunosuppression section) does not increase risk of skin cancers.
Therefore, transplant recipients who develop multiple skin cancers can be considered for a switch to a sirolimus-based, calcineurin-inhibitor free immunosuppression regimen. Currently, there is no data to indicate that liver transplant recipients are at increased risk to develop other common cancers such as breast, colon, prostate, or other cancers.
Back to top
Outcomes
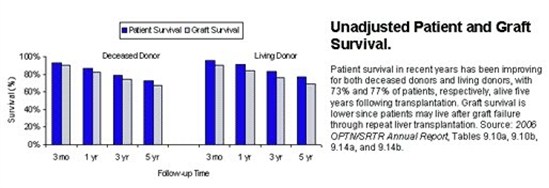
Overall, outcomes for liver transplantation are very good, but vary significantly depending on the indication for liver transplant as well as factors associated with the donor. Currently, the overall patient survival one year after liver transplant is 88%. Patient survival five years after liver transplant is 73%.
As mentioned above, these results vary significantly based on the indication for liver transplantation. For example, patients who underwent transplantation for hepatocellular carcinoma had a one-year survival of only 86% whereas patients who underwent transplantation for biliary atresia liver disease had a one-year survival of 94%. The encouraging trend is that over the past 20 years short and long term patient survival has continued to improve. With advances in surgical technique, organ preservation, peri-operative care, and immunosuppression, survival will hopefully continue to improve in the future.
Disclaimer: This article has been taken from https://transplantsurgery.ucsf.edu// as it is. Click here to read the original article.